Published on 11 September 2025
2024/1 GaBI Journal Table of Contents
24 views
Published on 11 September 2025
24 views
 Interview
Interview
Published on 15 April 2025
Author(s): GaBI Journal Editor
biopharma, biopharmaceutical, biosimilars, biotech, Turkey
451 views
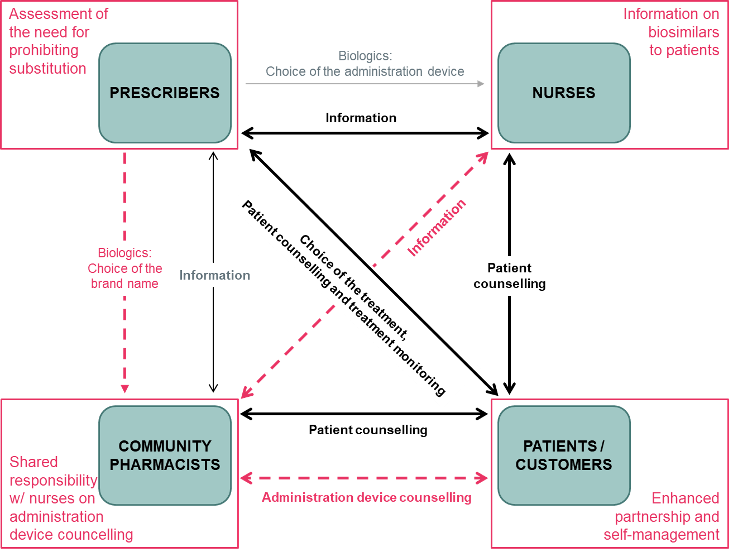 Meeting Report
Meeting Report
Published on 14 April 2025
Author(s): Professor Alan Lyles, BSPharm, MPH, ScD, PhD (h.c.), Heinonen E, MD, PhD, Tolonen HM, MSc (Pharm), PhD, Linden K, MSc (Pharm), MSc (Econ), PhD, Sihvo S, PhD, Sarnola K, MSc (Pharm), MSc (Econ), PhD, Airaksinen M, MSc (Pharm), PhD
affordable medicines, automatic substitution, biological medicines, biosimilar, community pharmacy, Finland
536 views
 Meeting Report
Meeting Report
Published on 25 March 2025
Author(s): Michael S Reilly, Esq, Ralph D McKibbin, MD, FACP, FACG, AGAF, Professor Philip J Schneider, MS, FASHP, FASPEN, FFIP, Andrew Spiegel, Esq
interchangeable biosimilar, misinformation, switch studies
521 views
Published on 17 January 2025
Author(s): Robin Thorpe, PhD, FRCPath
DOI: 10.5639/gabij.2024.1303.032
420 views
Published on 14 January 2025
biopharmaceutical, critique, interchangeable biosimilar, meta-analysis, misinformation
556 views
Published on 18 December 2024
Author(s): Adjunct Professor Pekka Kurki, MD, PhD
interchangeable biosimilar, misinformation, switch studies
546 views
 Review Article
Review Article
Published on 18 November 2024
Author(s): Vimal Sachdeva, MSc
authorization, best practice, harmonization, pharmaceuticals, recognition
DOI: 10.5639/gabij.2024.1303.034
1.942 views
Published on 07 November 2024
Author(s): GaBI Journal Editor
biosimilars, clinical development, commercialization, MENA (Middle East and North Africa)
DOI: 10.5639/gabij.2024.1303.037
906 views
Published on 06 May 2024
Author(s): Irene Krämer, PhD, Frank Erdnuess, PhD, Judith Thiesen, PhD
azacitidine, physicochemical stability, suspension for injection
DOI: 10.5639/gabij.2024.1302.023
1.157 views
Published on 11 September 2024
bortezomib, intravenous injection, physicochemical stability, subcutaneous injection
DOI: 10.5639/gabij.2024.1302.015
706 views
Published on 11 September 2024
cabazitaxel, concentrated solution, diluted infusion solution, HPLC, physicochemical stability
DOI: 10.5639/gabij.2024.1302.027
795 views